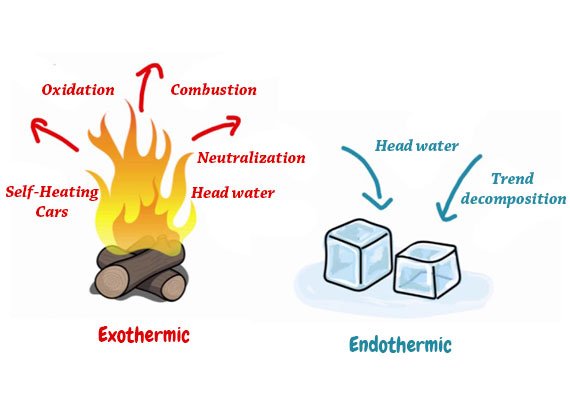
What is an Endothermic Reaction?
Products tend to be higher in energy compared to the reactants in the endothermic reaction. So, the alteration in enthalpy happens to be positive. Again, the heat gets absorbed by the reaction from the surroundings. Endothermic reactions lower their surrounding area’s temperature and so, become successful in forming a cooling effect. Some physical processes can become endothermic too like an ice cube absorbs heat energy from its surroundings. It also melts for forming liquid water.
We allow our students to pay our fees in installments and this impresses students to no end. This is one of the several reasons for which students count on our Exothermic and Endothermic Reactions assignment help online.
How Do Exothermic Reactions Differ from Endothermic Reactions?
The terms ‘Exo’ and ‘Endo’ possess Greek roots which means ‘out’ and ‘within’ respectively. According to the names, the chief difference between exothermic and endothermic reactions is the former includes the discharge of heat whereas the latter absorbs heat from their surroundings.
The human body does exploit the endothermic quality of evaporation for cooling itself and it is accomplished via the method of sweating. The sweat which is produced on one’s skin’s surface absorbs heat for evaporating and it results in a cooling effect. Nonetheless, sweating isn’t viewed as an exothermic reaction and chemical reactions can include the formation of fresh bonds or the breaking of the current chemical bonds, and at times, both. The sweat’s evaporation doesn’t include any chemical alterations but it surely does include an alteration in the physical phase, which is vapor from the liquid.
So, evaporation can be classed as a physical endothermic process in place of an endothermic reaction. The processes that absorb heat from their surroundings are known as endothermic processes and so, all the endothermic reactions tend to be endothermic processes, though the opposite isn’t true. Numerous endothermic processes include physical alterations in place of chemical alterations.
Students always come to us for getting Exothermic and Endothermic Reactions homework writing service from us because we never copy content from other sources but do extensive research on every topic ourselves.





 3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029
3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029
